Transition Metals
Transition metals can be split into the following sections
- General properties
- Ligand Substitution reactions
- Isomerism of complex ions
- Formation of coloured ions
- Variable oxidation states
- Catalysis
General Properties
Transition metals are d-block metals which is an atom or has a stable ion with an incomplete d subshell.
Iron(III): 1S22S22P63S23P63d5
Note the d subshell as 5 electrons out of a possible 10, this confirms iron is a transition metal.
Zinc: 1S22S22P63S23P63d104S2
Zinc(II): 1S22S22P63S23P63d10
The Zinc atom and its only stable ion has a complete d subshell, this means that zinc is not a transition metal, but it is a d-block metal. Transition metal ions will produce complex ions these are written in [square brackets] with he overall charge. A complex ion is one which has coordinate bonds to ligands, a coordinate bond is one in which a bond is formed from two electrons from the same species, the amount of coordinate bonds to a complex ion is known as its coordinate number. A ligan is a molecule or ion which provides the coordinate bond to the complex ion, typical mono-dentate ligands are;
- CN-
- H2O
- NH3
- Cl-
- OH-
There is 4 shapes of complex ions we need to be aware of
Linear often silver containing e.g. [AgCl2]-
Square planar often platinum containing e.g [Pt(NH3)4]2+
Tetrahedral often containing chloride ions e.g [CuCl4]2-
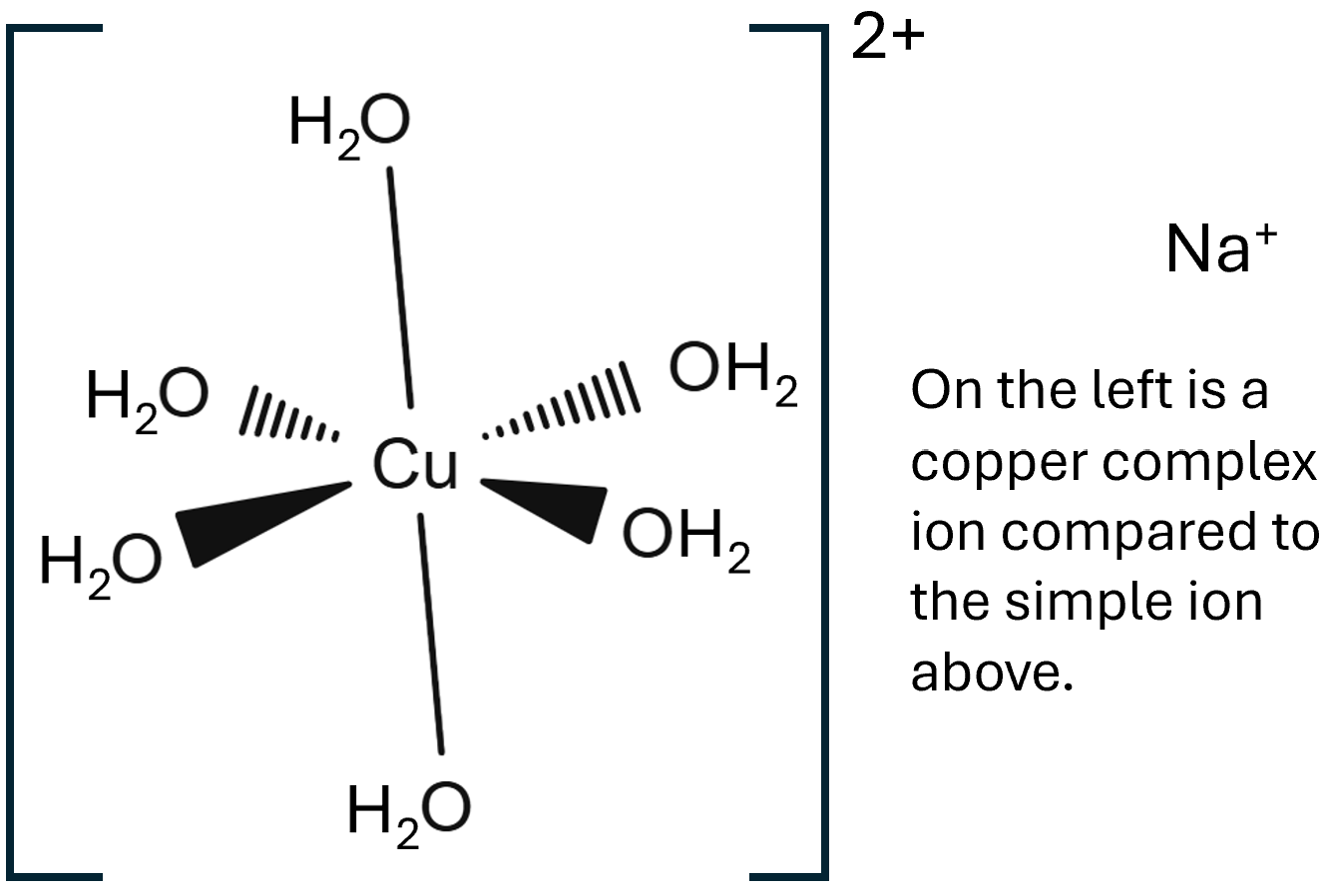
Octahedral this is the most common shape you will experience e.g [Cu(H2O)6]3+
Ligand Substitution Reactions
There are several monodentate, single bite, ligands which all provide one covalent bond. Bidentate, two bites, each provide 2 coordinate bonds to the transition metal and multidentate ligands will provide many coordinate bonds.
Ligands can be changed in a ligand exchange reaction for example
[Co(H2O)6]+2 +6NH3 → [Co(NH3)6]+2 + 6H2O
In this ligand exchange reaction the coordination number stays at 6 as there is 6 coordinate bonds to the cobalt ion in the reactants and product, this is because NH3 and H2O are similar sized ligands. The ligand exchanges could be complete or incomplete
Complete ligand exchange [Co(H2O)6]+2 +6NH3 → [Co(NH3)6]+2 + 6H2O
Incompelte ligand exchange [Co(H2O)6]+2 +4NH3 → [Co(NH3)4(H2O)2]+2 + 4H2O
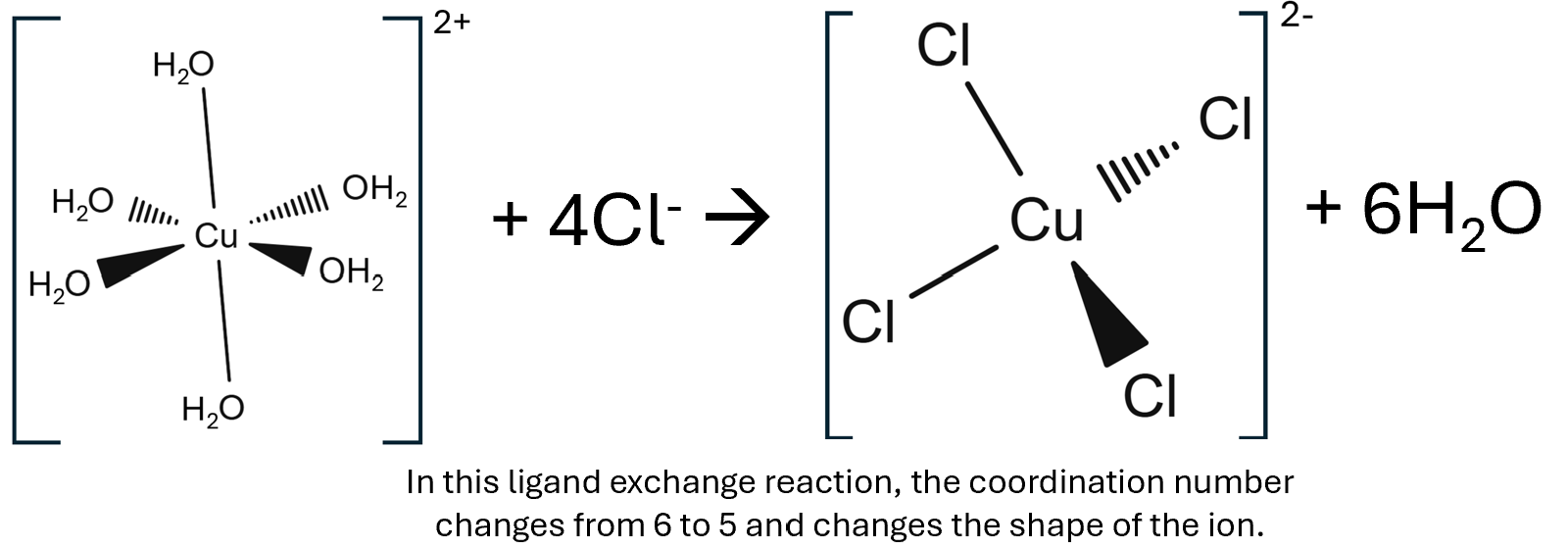
The chloride ligand is larger than ammonia and water ligand, when it substitutes these in a ligand exchange due to chlorides size only four of these ligands can fit around the tranisiton metal ion reducing its coordination number from 6 to 4.
[Cu(H2O)6]2+ + 4Cl- → [CuCl4]2- + 6H2O
The common bidentate ligands are
- Ethandioate
- Ethane-1,2-diamine
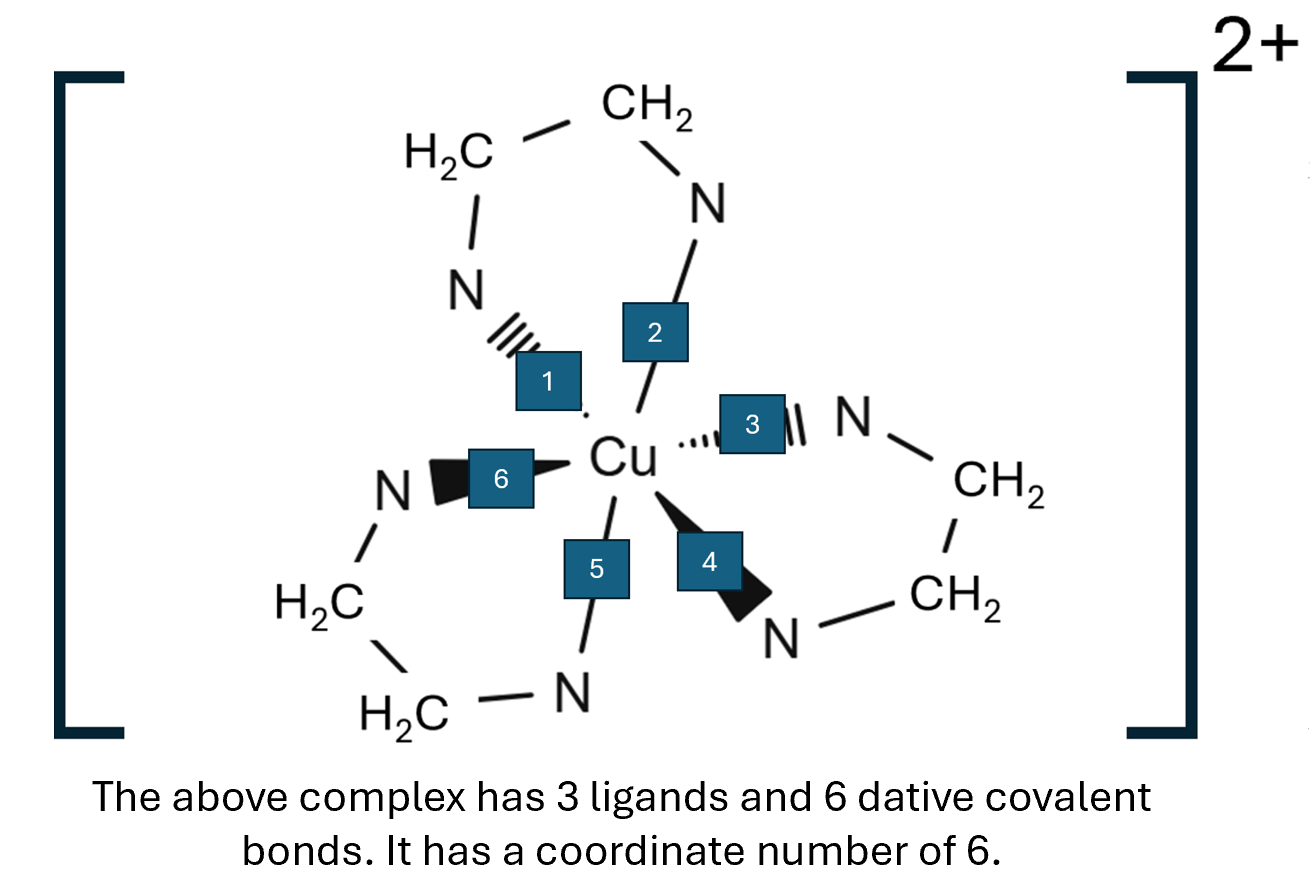
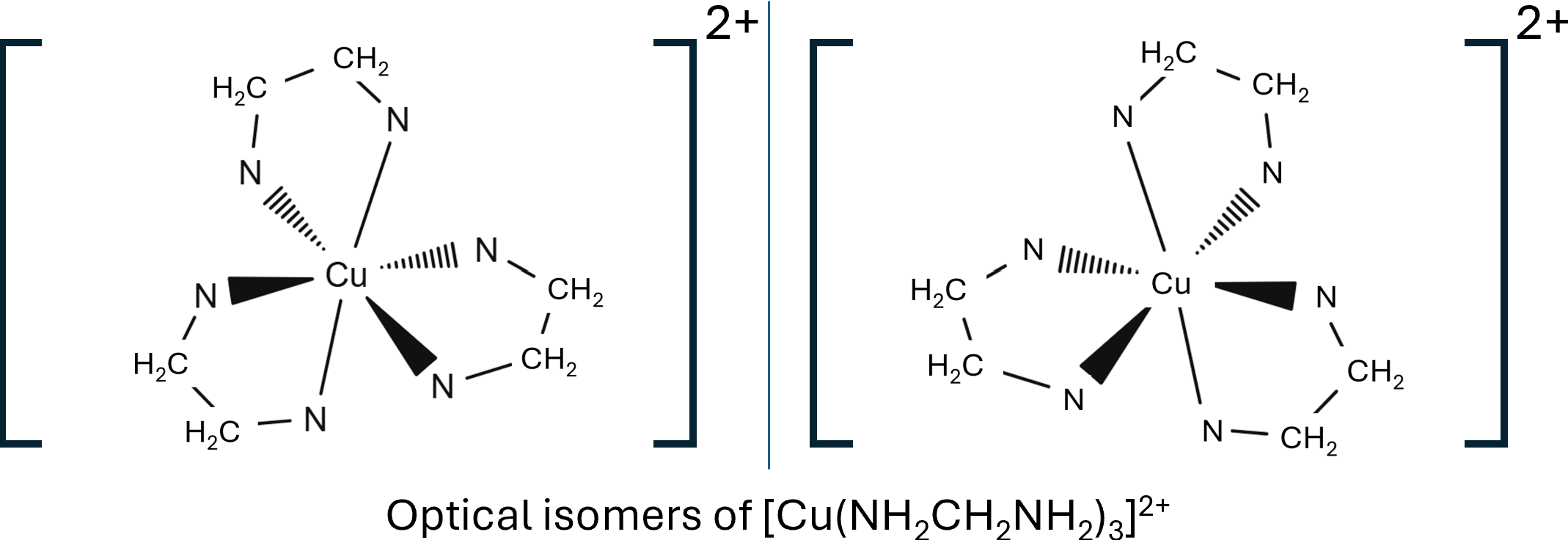
The coordination number of a complex ion is dictated by the amount of coordinate bonds, not the number of ligands so [Co(NH2C2H4NH2)3]2+ would have a coordination number of six.
The chelate effect
The chelate effect is the affect of entropy on driving ligand substitution reactions. Gibb’s free energy will dictate if a reaction is feasible or not, with many of the ligand substitution reactions there is little change in enthalpy, this can be shown below where a coordinate bond between the cobalt ion and oxygen in water is replace with a bond between the cobalt ion and oxygen in ethandioate.
With ΔH being close to zero the reaction must be driven by a change in entropy, with the below reaction using a multidentate ligand the number of species in the reactants is 2 and in the products it is 7, with such a large increase in the number of particles there is a significant increase in entropy providing the driving force for the reaction. [Co(H2O)6]2+ + EDTA4- → [CoEDTA]-2 + 6H2O
Haem is a complex ion contained Iron (II) and a multidentate ligand based on a porphyrin ring.
Oxygen will form a coordinate bond to the iron ion and be carried about the blood stream, carbon monoxide, another monodentate ligand will also create a coordinate bond to this iron ion in place of oxygen, but the body will not be able to remove it reducing the ability of the haem to carry oxygen around the body causing illness and death.
Isomerism of complex ions
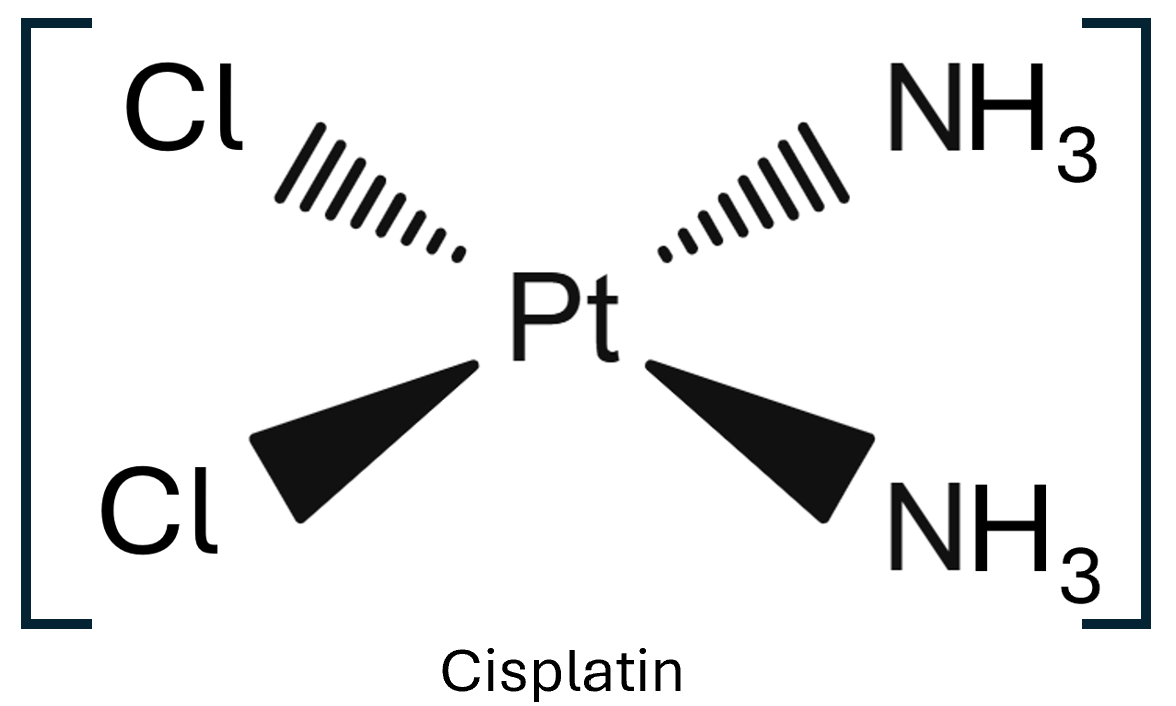
Cisplatin is an anticancer drug, it’s formula is [Pt(NH3)2Cl2]. This square planar complex ion has two stereoisomers, cis and trans. The cis isomer, cisplatin, has the same ligands next to each other, whereas transplatin has the same ligands opposite to each other.
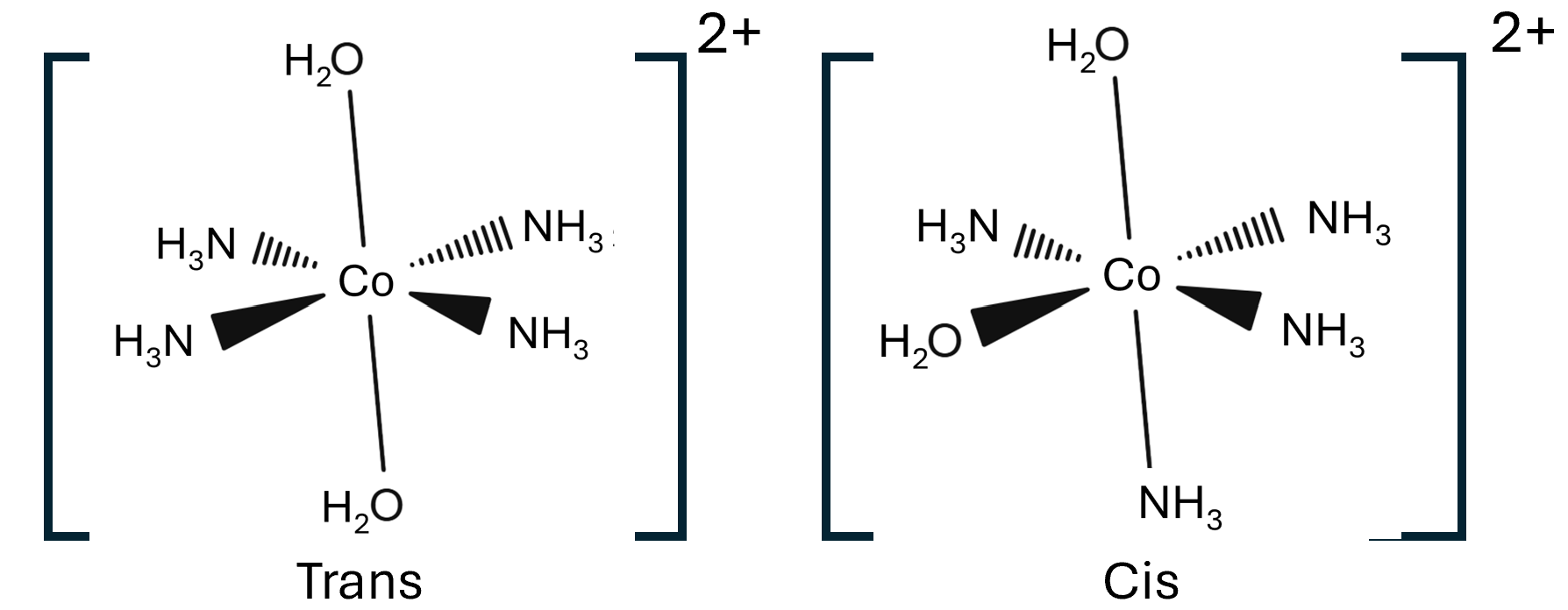
Octahedral complex ions can also exhibit cis and trans isomerism for example the below is [Cu(NH3)4(H2O)2]2+.
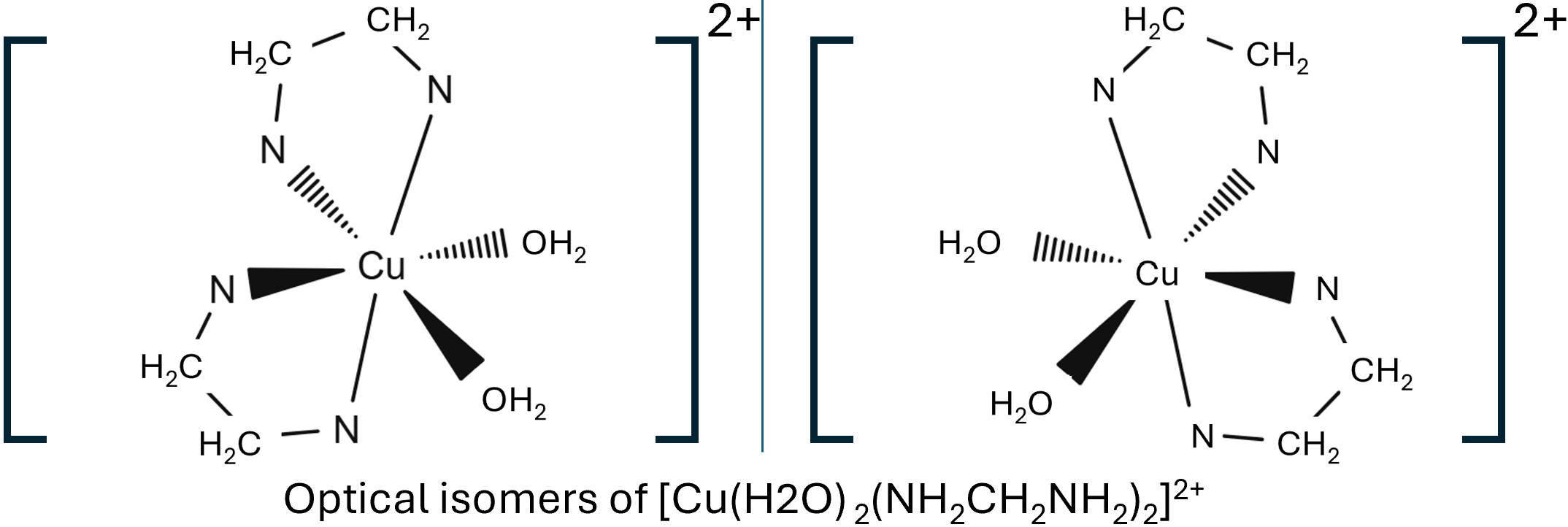
The cis isomer has the two water ligands at 900 to each other, where as the trans isomer has the two water ligands at 1800 to each other. Octahedral complex ions can also show optical isomerism, non-superimposable mirror images, fs there is 2 or 3 bidentate ligands, these are molecules you should practice drawing.
Formation of coloured ions
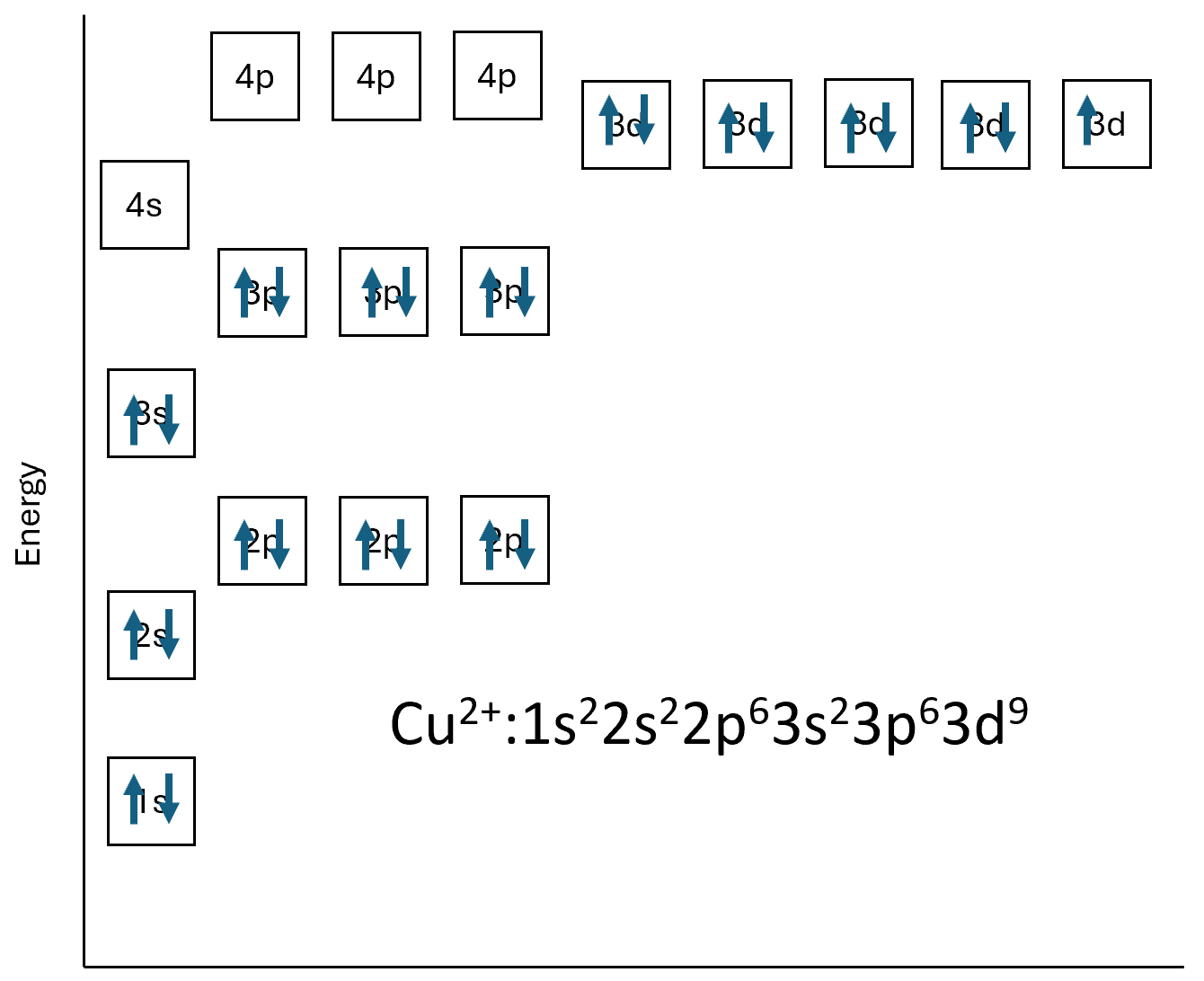
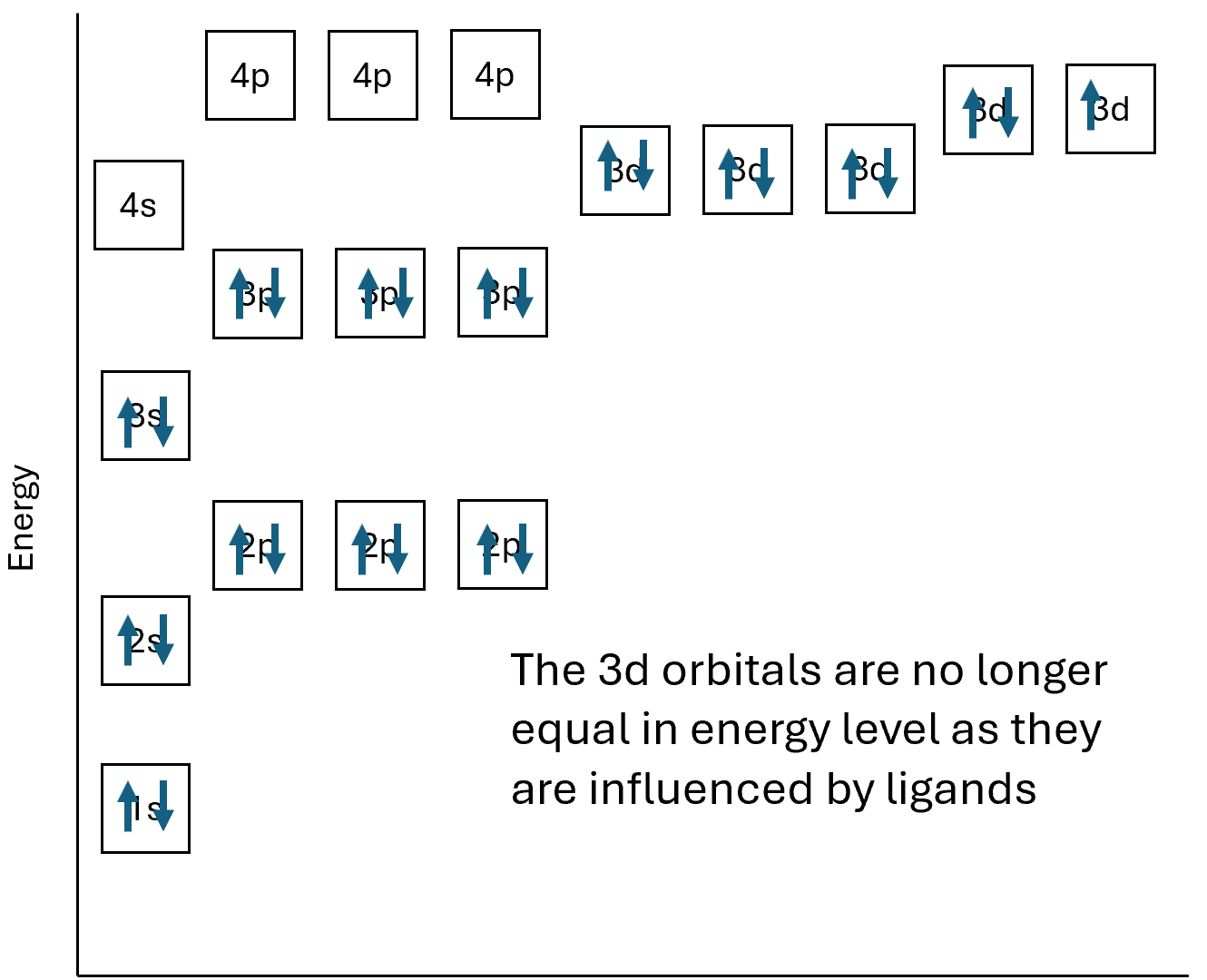
Transition ions produce coloured solutions and compounds because of having incomplete d subshells. Previously when we have looked at electron structure it has been of single atoms or ions, the below is the electronic structure of Cu2+.
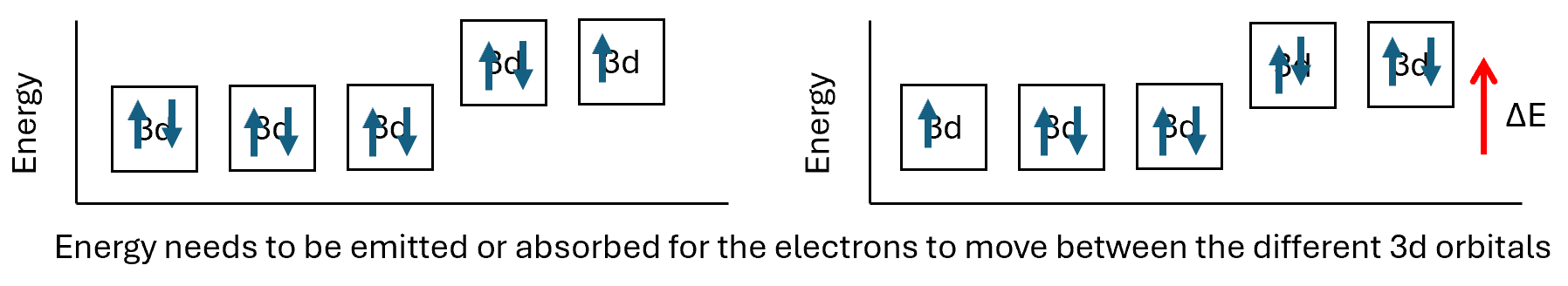
The d subshells all have the same energy level as each other, if an electron was to move between the d orbitals there would be no change in energy. When in a complex ion the electrons from the coordinate bond will affect the energy levels of the d subshell and the electron structure diagram would be different.
As you can now see the d orbitals are no longer the same energy level, the ions can now absorb energy which corresponds to electrons moving within the d subshell. The energy which is absorbs corresponds to a photon, a packet of light, which is in the visible spectrum. We can calculate the energy using the equation ΔE = hc/λ where E is energy, h is Planck’s constant, c is the speed of light and λ is the wavelength.
For example we could calculate the energy required to excite one electron in [CuCl4]2-. The yellow colour is formed from the solution absorbing colours other than yellow. Light of wavelength 750nm is absorbed.
ΔE = hc/λ
ΔE is the unknown
h = 6.62x10-34
c = 3x108m/s
λ = 450nm = 4.5x10-7m
ΔE = 6.62x10-34 x3 x108 / 4.5x10-7 = 4.42x10-19 J
The amount of energy, and therefore colour of the ion can be changed by several factors;
- Metal ion
- Oxidation state of metal ion
- The type of ligand
- The coordination number of the complex ion
Variable Oxidation States
Transition metals will have several ions with different oxidation states. These can be demonstrated by redox titrations, most of these titrations will be self-indicating and not need an indicator adding. The examples that you will need to know are,
5Fe2+ + MnO42- + 8H+ → 5Fe3+ + Mn2+ + 4H2O
5C2O42- + 2MnO42- + 16H+ → 10CO2 + 8H2O + 2Mn2+
In both reactions the end point is identified by the colour change of the manganese ion, when in its +7 state it is deep purple, and the +2 ion is colourless. There is also the requirement of being an acidified solution, the decrease in pH increase the redox potential of the ions. If the solution is not acidified no reaction occurs.
Vanadate (v) can also be oxidised to several different oxidation states by zinc in an acidic solution which is expressed in the following reactions
VO2+ + 2H+ + e- → VO2+ + H2O
VO2+ + 2H+ + e- → V3+ + H2O
V3+ + e- → V2+
Overall VO2+ + 4H+ + 3e- → V2+ + 2H2O
When combining with the zinc half equation
Zn → Zn2+ + 2e-
1½Zn + VO2+ + 4H+ → 1½Zn2+ + V2+ + 2H2O
Catalysis
Transition metals are often used as catalysts in both heterogenous, different phase, and homogeneous, same phase, catalysis.
Examples of heterogenous catalysis include,
- Iron for the haber process
- Platinum/Palladium/Rhodium for catalytic convertors
- Zeolite for cracking
- Vanadium (V) Oxide for the contact process
For the first 3 of the above we can explain the process in the same way with adsorption, rection and then desorption.
- The reactants adsorp on to the surface of the catalyst, this weakens the bonds within the molecules
- The reactants with weakened bonds have their activation energy reduced and the reaction takes place.
- The products now desorp from the surface of the catalyst
Heterogenous catalysts work with an active site, if the active site reacts with another compound it is known as poisoned as does not work or has reduced efficiency. The catalyst will need to be replaced or regenerated which has a cost implication. The reaction of SO2 to SO3 catalysed by V2O5 for the contact process, production of sulfuric acid, is one which you need to know.
SO2 + V2O5 → SO3 + V2O4
V2O4 + ½ O2 → V2O5
A homogenous catalyst is one which is in the same phase as the reactants, some examples of this include,
- I- and S2O82- catalysed by Fe2+
- C2O42- and MnO4- catalysed by Mn2+
In both of these reactions the two reactants are negatively charged meaning the activation energy of the reaction is high. The catalyst introduces an intermediate step which avoids the requirement of two negative ions reacting.
S2O82- + 2I- → 2SO42-+ I2
In the overall reaction above the S2O82- ion and I- ion would both repel each other, with a catalyst we get the following two reactions
S2O82- + 2 Fe2+ → 2SO42- + 2Fe3+
2Fe3+ + 2I- → 2 Fe2+ + I2
In the catalysed reaction the reactions occur between oppositely charged ions which will attract each other and have a significantly reduced activation energy.
For the reaction between C2O42- and MnO4- catalysed by Mn2+, the product is a catalyst for the reaction which is known as autocatalysis which means the rate significantly increases as time goes on.
Overall 2MnO4- + 16H+ + 5C2O42- → 2Mn2+ + 8H2O + 10CO2
Catalysed 4Mn2+ + MnO4- + 8H+ → 5Mn3+ + 4H2O
2Mn3+ + C2O42- → 2CO2 + 2Mn2+
In the overall reaction the MnO4- ions and C2O42- ions repel each other as they are both negative, in the catalysed reaction the Mn2+ and MnO4- react with each other first with a lower activation energy as they are not repelling each other, they are attracting each other. In the second catalysed reaction the Mn3+ ions then react with the C2O42- ions which again has a low activation energy as they are oppositely charged.