Reactions of ions in aqueous solutions
The reactions of ions in aqueous solutions can be broken down in to the following
- Complex ions of 2+ and 3+ oxidation states
- Reactions of complex ions with OH-
- Reactions of complex ions with NH3
- Reactions of complex ions with CO32-
- Amphoteric Nature of Al complex ions
- Acidic nature of 3+ complex ions.
Complex ions of 2+ and 3+ oxidation states;
The ions within the specification for this section are
[Fe(H2O)6]2+
[Cu(H2O)6]2+
[Fe(H2O)6]3+
[Al(H2O)6]3+
Another complex ion which is seen in other parts of the specification include [Co(H2O)6]2+ so within these notes cobalt complex chemistry will be included.
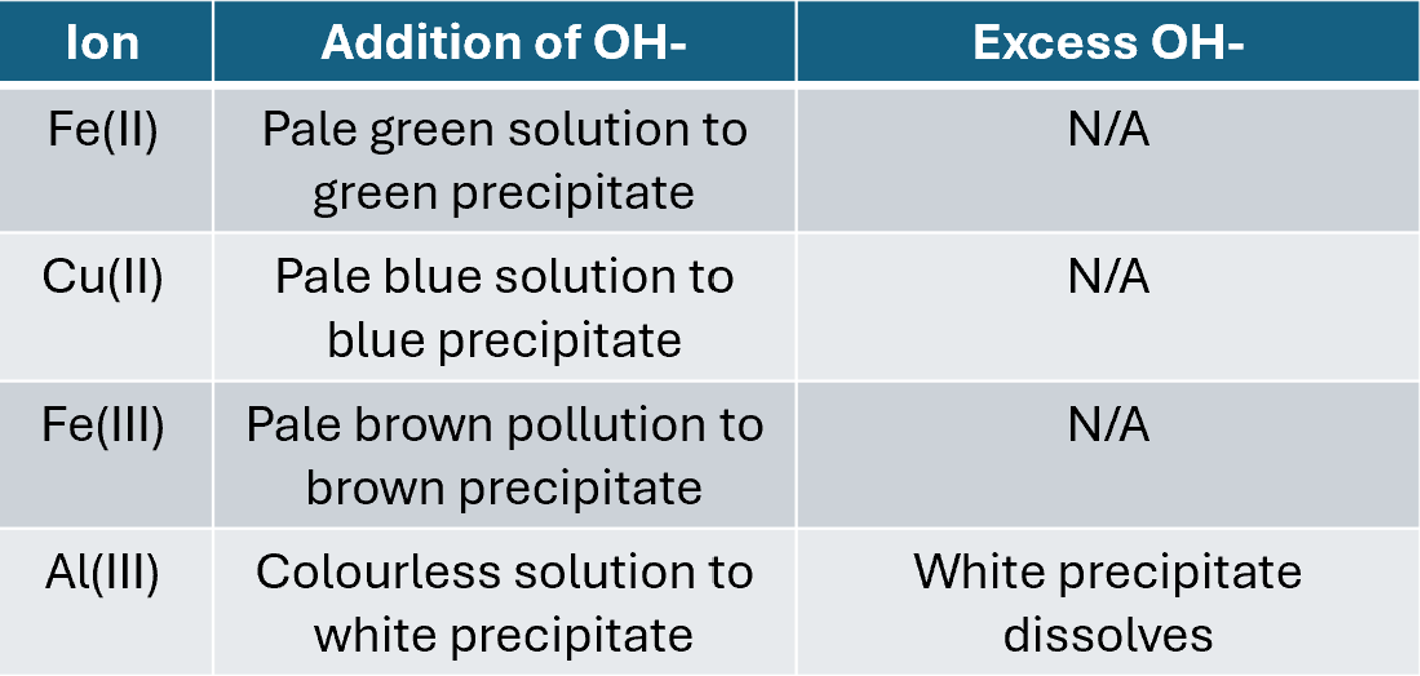
Reactions of complex ions with hydroxide
In all of the below reactions the complex ion will act as an acid and donate protons to the hydroxides until the complex ion becomes neutral and precipitates, aluminium is a exception as It can redissolve with excess hydroxide.
[Fe(H2O)6]2+(aq) + 2OH-(aq) → [Fe(H2O)4(OH)2] (s) + 2H2O(l)
Observation: Pale green solution to green precipitate
[Cu(H2O)6]2+(aq) + 2OH-(aq) → [Cu(H2O)4(OH)2] (s) + 2H2O(l)
Observation: Pale blue solution to pale blue precipitate
[Fe(H2O)6]3+(aq) + 3OH-(aq) → [Fe(H2O)3(OH)3] (s) + 3H2O(l)
Observation: Pale brown solution to drown precipitate
[Al(H2O)6]3+(aq) + 3OH-(aq) → [Al(H2O)3(OH)3] (s) + 3H2O(l)
Observation: Colourless solution to white precipitate
On the addition of excess hydroxide ions
[Al(H2O)3(OH)3] (s) + OH- → [Al(OH)4]- (aq)+ 3H2O(l)
Observations: White precipitate dissolves
Reactions of complex ions with ammonia
In the below reactions either there is ligand exchange where a water ligand is replaced with an ammonia, in the case of copper and cobalt, or a proton transfer where the ammonia acts as a base and the complex ion acts as an acid.
[Co(H2O)6]2+(aq) + 6NH3(aq) → [Co(NH3)6]2+(aq) + 6H2O(l)
Observation: Solution turns from pink to straw yellow
[Fe(H2O)6]2+(aq) + 2NH3(aq) → [Fe(H2O)4(OH)2] (s) + 2NH4+(aq)
Observation: Pale green solution to green precipitate
[Cu(H2O)6]2+(aq) + 2NH3(aq) → [Cu(H2O)4(OH)2] (s) + 2NH4+(aq)
Observation: Pale blue solution to blue precipitate
On the addition of excess ammonia
[Cu(H2O)6]2+(aq) + 4NH3(aq) → [Cu(NH3)4(H2O) 2]2+(aq) + 4H2O (l)
Observation: pale blue solution to blue precipitate which redissolves to form deep blue solution
[Fe(H2O) 6]3+(aq) + 3NH3(aq) → [Fe(H2O)3(OH)3] (s) + 3NH4+(aq)
Observation: Pale brown solution to brown precipitate
[Al(H2O) 6]3+(aq) + 3NH3(aq) → [Al(H2O)3(OH)3] (s) + 3NH4+(aq)
Observation: Colourless solution to white precipitate
Reactions of complex ions with carbonate ions
When carbonate ions react with complex ions they either produce a solid carbonate precipitate if it is a +2 metal ion or an acid-base reaction occurs if it is a +3 metal ion.
[Fe(H2O) 6] 2+(aq) + CO32- (aq)→ FeCO (s) + 6H2O (l)
Observation: Pale green solution to green precipitate
[Cu(H2O) 6] 2+(aq) + CO32- (aq)→ CuCO (s) + 6H2O (l)
Observation: Pale blue solution to green-blue precipitate
[Fe(H2O) 6] 3+(aq) + 1½CO32- (aq)→ [Fe(H2O)3(OH)3] (s) + 1½H2O (l) + 1½CO2(g)
Observation: Pale brown solution to brown precipitate and bubbling
[Al(H2O) 6] 3+(aq) + 1½CO32- (aq)→ [Al(H2O)3(OH)3] (s) + 1½H2O (l) + 1½CO2(g)
Observation: Colourless solution to white precipitate and bubbling
Amphoteric properties of aluminium complexes
The reaction of aluminium complex ions with hydroxide will produce the complex [Al(H2O)3(OH)3] which will react with an acid and act as a base for example
[Al(H2O)3(OH)3] (s) + 3HCl(aq) → [Al(H2O)6]3+(aq) + 3Cl-(aq)
The same complex will also react with a base as previously seen above.
[Al(H2O)3(OH)3] (s) + NaOH(aq) → Al(OH)4-(aq) + Na+(aq)
As this aluminium complex can act as both an acid and a base it is known as amphoteric.
Acidic nature of M3+ ions
In both of the previous reacts with M3+ ions and carbonate ions there is an acid-base rection instead of a ligand exchange reaction. This occurs because the M3+ ion is smaller than the M2+ ion and has a higher charge density, this withdraws more electron density from the oxygen within the ligand and weakens the covalent bond between the oxygen and the hydrogen in the water ligand, the weaker bond means that the proton requires less energy to dissociate into an H+ ion.