Period 3
Properties of period 3 elements and their oxides can be broken down into the following parts
- Elements Reactions with H2O
- Elements reactions with H2O
- Elements reactions with O2
- Structure and Bonding of the Oxides
- Oxides reactions with H2O
- Oxides reactions with Acids and Bases
- Oxides reacting as bases
- Oxides Reacting as acids
Elements reactions with H2O
Sodium will react with water in the below reaction
Na(s) + H2O(l) → NaOH + ½ H2(g)
The reaction is exothermic; this will melt the sodium which will be floating on the surface of the water due to its low density. The sodium will seem to disappear as it reacts to become sodium hydroxide and dissolve into the water, a gas will be produced which could be collected and then confirmed by the squeaky pop test to be hydrogen.
Magnesium’s reaction with water is significantly slower again producing a hydroxide and hydrogen gas but taking many days to fill a test tube with gas.
Mg(s) + 2H2O(l) → Mg(OH)2(aq) + H2(g)
The NaOH solution will high highly alkaline, the Mg(OH)2 solution is less alkaline even though there is more hydroxide ions in the compound as Mg(OH)2 is sparingly soluble and little will dissolve in the water.
Magnesium will react much more vigorously with steam and produce a different reaction.
Mg(s) + H2O(g) → MgO(s) + H2(g)
The observations would be of a bright white light and then white powder forming.
Elements Reactions with O2
Sodium will burn with a yellow flame in oxygen and produce white sodium oxide.
4Na(s) + O2(g) → 2Na2O(s)
Magnesium will burn with a white flame in oxygen and produce white magnesium oxide.
2Mg(s) + O2(g) → 2MgO(s)
Aluminium will burn with a white flame in oxygen to produce white aluminium oxide. Most aluminium will have a very thin layer of aluminium oxide on its surface which act as a barrier stopping the aluminium below it from further reacting with oxygen in the air.
4Al(s) + 3O2(g) → 2Al2O3(s)
Silicon will react with oxygen when heated strongly to produce silicon dioxide, pure silicon dioxide is white, but impure silicon dioxide is coloured.
Si(s) + O2(g) → SiO2(s)
Phosphorus is in several allotropes, red and white most commonly. Red phosphorus will burn with a pink flame when heated in oxygen, white phosphorus will spontaneously ignite in oxygen with a pink flame.
4P(s) + 5O2(g) → P4O10(s)
Sulfur will burn with a blue flame in oxygen producing sulfur dioxide.
S(s) + O2(g) → SO2(g)
The sulfur dioxide can then react with oxygen to form sulfur trioxide; in industry a vanadium (V) oxide catalyst is used.
2SO2(g) + O2(g) → 2SO3(g)
Structure and bonding of the oxides of period 3
Sodium Oxide, Magnesium Oxide, and Aluminium Oxide are all giant ionic structures, aluminium oxide shows the most covalent characteristics of these three oxides.
Magnesium oxide has a higher melting point than sodium oxide due to the magnesium ion forming a smaller cation, this smaller cation can form a stronger electrostatic force to the oxide ion, which would require more energy to overcome and a higher melting point.
Aluminium oxide with the above reasoning should have a higher melting point than magnesium oxide, unfortunately there is other affects in play which mean the melting point of aluminium oxide is less than magnesium oxide but above sodium oxide.
Silicon dioxide is the only giant covalent oxide of period 3, it has a relatively high melting point of 1713 oC.
The remainder of period 3 oxides, P4O10, SO2, and SO3, are simple molecular so have relatively low melting points and the large molecules having larger van der walls have higher melting points.
Oxide Reactions with water
Sodium oxide will react with water to form sodium hydroxide producing an alkaline solution of approximately pH 14.
Na2O(s) + H2O(l) → 2NaOH(aq)
Magnesium Oxide will react with water to form magnesium hydroxide, which is less soluble than sodium hydroxide so the solution will have an approximate pH of 9.
MgO(s) + H2O(l) → Mg(OH)2(aq)
The basic nature of sodium oxide and magnesium oxide is due to the presence of an oxide ion, the oxide ion will react with water to produce hydroxide ions.
Aluminium oxide and silicon dioxide and insoluble in water and do not react with water.
Aluminium oxide is classed as an ionic lattice but due to an increased amount of covalent characteristics it is insoluble and therefore does not provide aqueous oxide ions. Silicon dioxide is a giant covalent structure so does not have oxide ions present.
O2-(aq) + H2O(l) → 2OH-(aq)
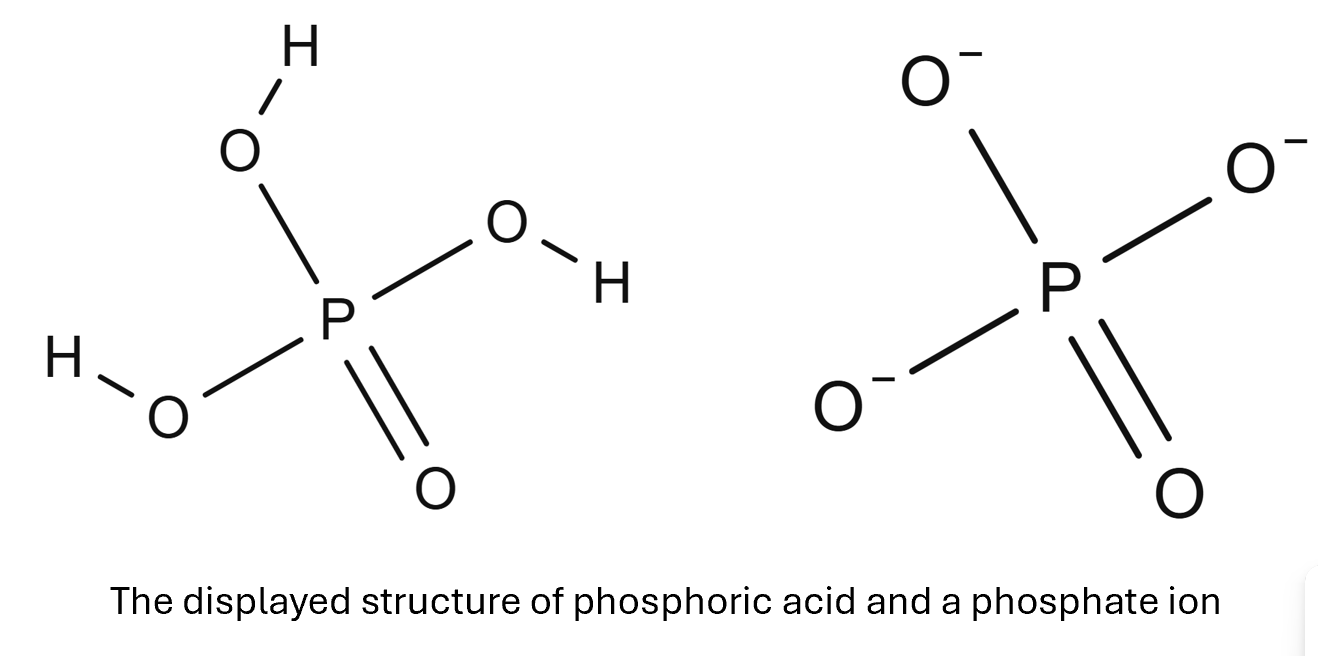
Phosphorus (V) Oxide will react with water to form phosphoric acid producing an acidic solution of approximately pH 1.
P4O10(s) + 6H2O(l) → 4H3PO4(aq)
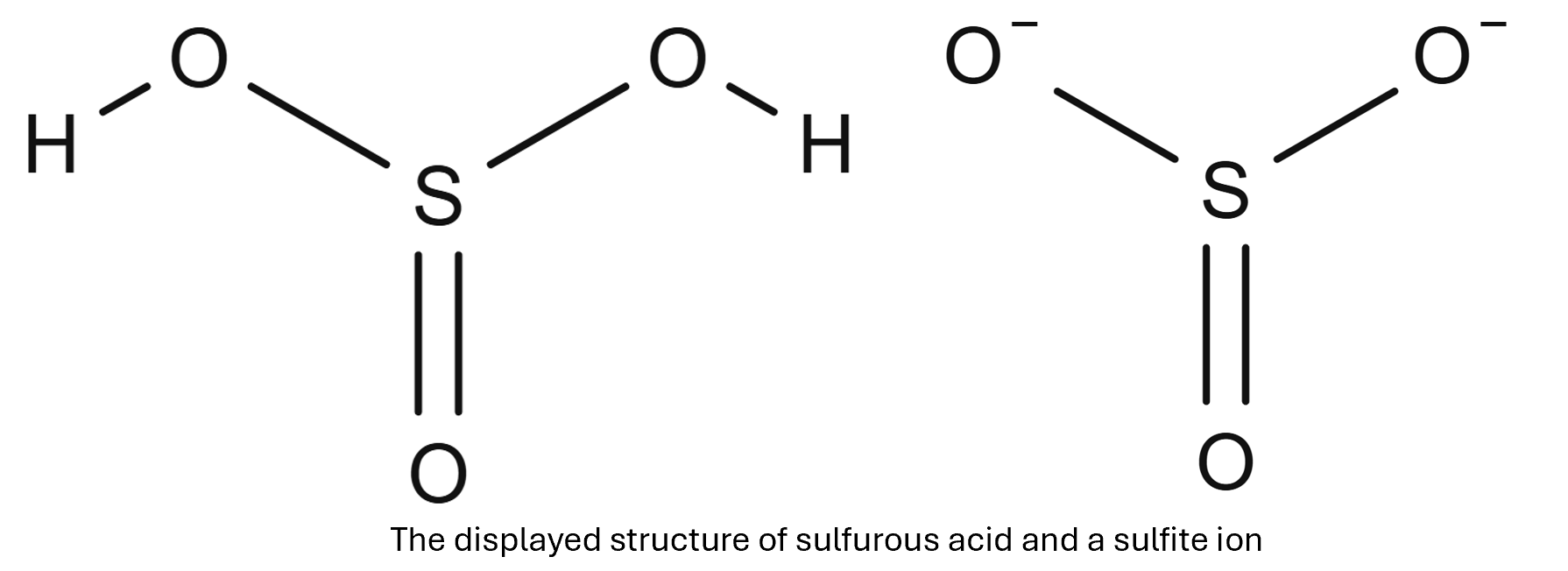
Sulfur dioxide will react with water to produce sulfourous acid, a weak acid, with an approximat pH of 2.
SO2(g) + H2O(l) → H2SO3(aq)
Sulfur trioxide will react with water to produce sulfuric acid, a strong acid, with an approximate pH of 0.
SO3(g) + H2O(l) → H2SO4(aq)
Oxides reacting as bases
Sodium oxide, magnesium oxide, and aluminium oxide all contain oxide ions, these oxide ions will accept two protons generating water, and the metal cations from the metal oxide will form salts with the conjugate bases.
O2-(aq) + 2H+(aq) → H2O(l)
Na+(aq) + Cl-(aq) → NaCl(aq)
Na2O(s) + 2HCl(aq) → NaCl(aq)
It is important to note that aluminium oxide even though insoluble does contain oxide ions so will react in a similar fashion.
O2-(s) + 2H+(aq) → H2O(l)
Al3+(s) + Cl-(aq) → AlCl3(aq)
Al2O3 + 6HCl(aq) → 2AlCl3(aq)
Period 3 Oxides as acids
Aluminium oxide can also act as an acid, this means it is amphoteric. An example of aluminium oxide reacting with hot concentrated sodium hydroxide is below.
Al2O3 + NaOH + 3H2O → 2NaAl(OH)4(aq)
Silicon dioxide will act as an acid and react with strong bases, for example
SiO2 (s) + 2NaOH(aq) → Na2SiO3(aq) + H2O(l)
Phosphorus Oxide, and both sulfur oxides when acting as bases will essentially react with water creating the acids mentioned previously and have predictable acid base reactions.
H3PO4(aq) + 3NaOH(aq) → Na3PO4(aq) + 3H2O(l)
H2SO3(aq) + 2NaOH(aq) → Na2SO3(aq) + 2H2O(l)
H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)